Investing in BiomeBank
An Overview
Page Contents
BiomeBank’s multi-disciplinary team of microbiome experts has now successfully developed a breakthrough co-culturing platform, ConsortiomeTM that allows us to produce a range of complex synthetic bacterial communities that can replicate the current donor-derived microbial therapies. However, because these new co-cultured therapies are produced in a bioreactor, they can be manufactured at a much lower cost and more easily scaled than donor-derived treatments.
More importantly these new generation of therapies have the potential to be enriched for defined and patented disease specific functions and used to treat the unmet medical needs of patients globally.
OVERLAP OF GENE FAMILIES BETWEEN
HEALTHY DONORS & BIOMEBANK’S CONSORTIOME™
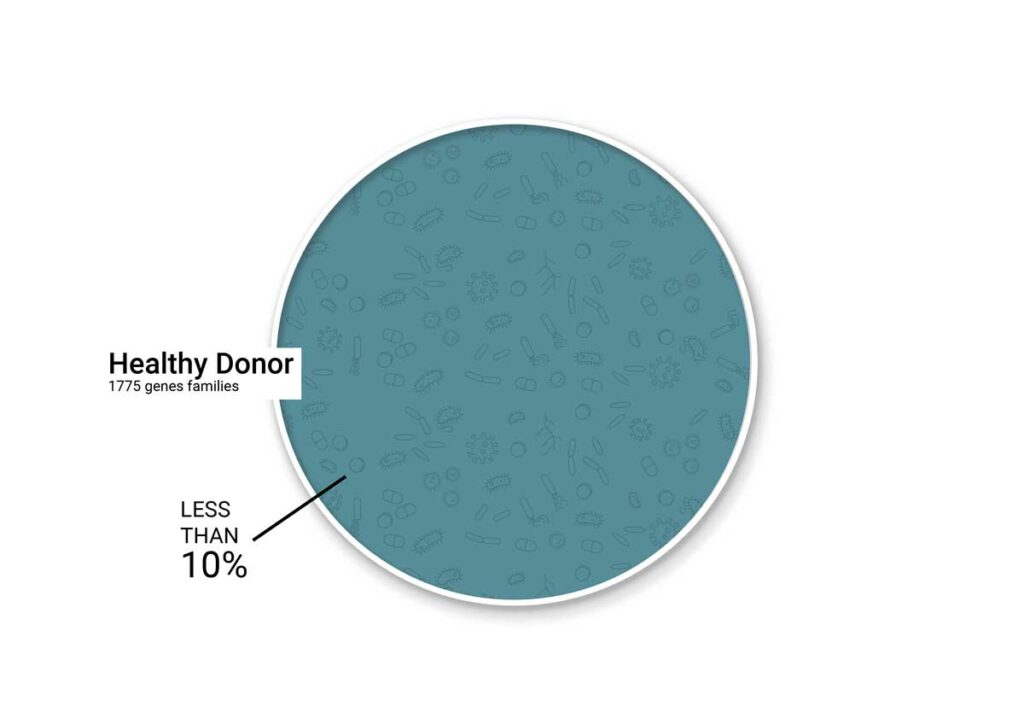
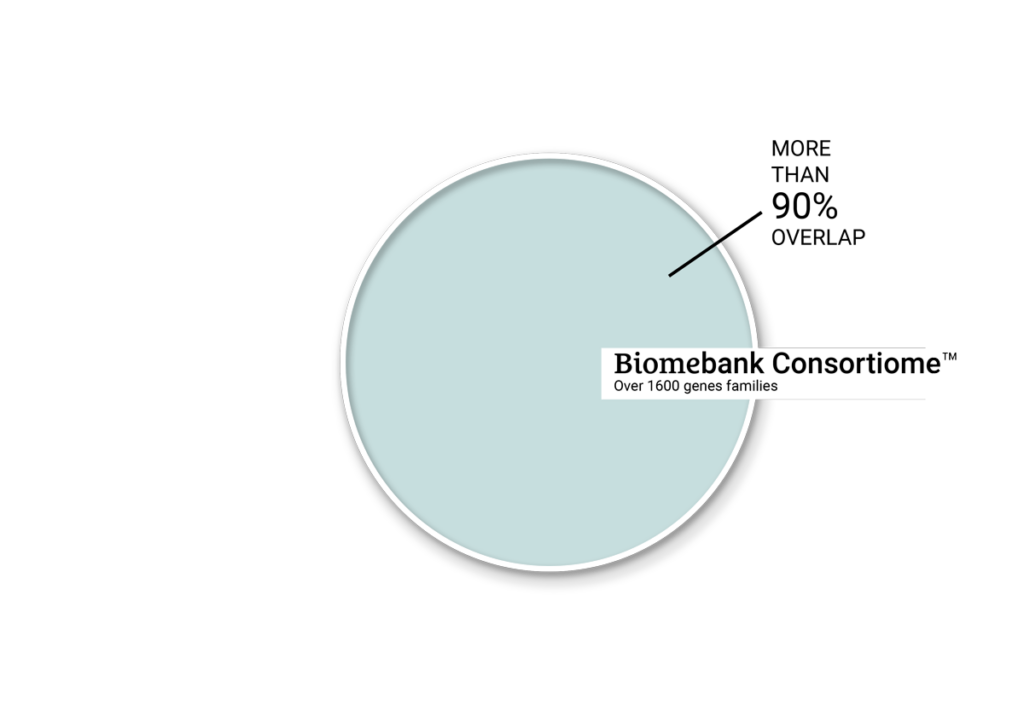
Annotation using the NCBI COG database (”Clusters of Orthologous Genes”) identified members of 1775 gene families from a healthy donor metagenome, of which over 90% of gene families were represented in the consortium metagenome.
Having observed the Company’s expertise in developing live biotherapeutic products to treat the unmet need of patients, I believe we’re in a strong position to improve the quality of life for people around the world as well as continue to build our business as a global biotechnology leader.
- Chairman Chris Hall
different?
-
Ecosystem Approach
A unique approach to microbiome-based therapies that treats disease by repopulating missing microbes and restoring lost functions
-
Experienced Team
BiomeBank is made up of a team of microbiome drug development experts based in a world-class cGMP manufacturing facility in the heart of Adelaide’s medical precinct
-
Approved Live Biotherapeutic Product
Our approved therapy for recurrent C.difficile infection shows a track record for commercialising microbial treatments
-
Unique Co-culturing Platform
Our breakthrough co-culturing platform gives us the potential to create treatments for defined and patented disease specific functions that have total addressable market sizes in the billions of dollars
The Market for Microbial Therapies
The global microbial therapeutic market is projected to reach a valuation of US$30 billion by 2030, making the gut microbiome a new frontier in medicine.
BiomeBank’s breakthrough co-culturing technology mean it is well-positioned to become a global leader in the sector. Our first such co-cultured therapy, BB265 for Ulcerative Colitis, has been designed using data from use of BiomeBank’s donor derived therapy in clinical trials and will enter human trials in 2024.
Ulcerative Colitis
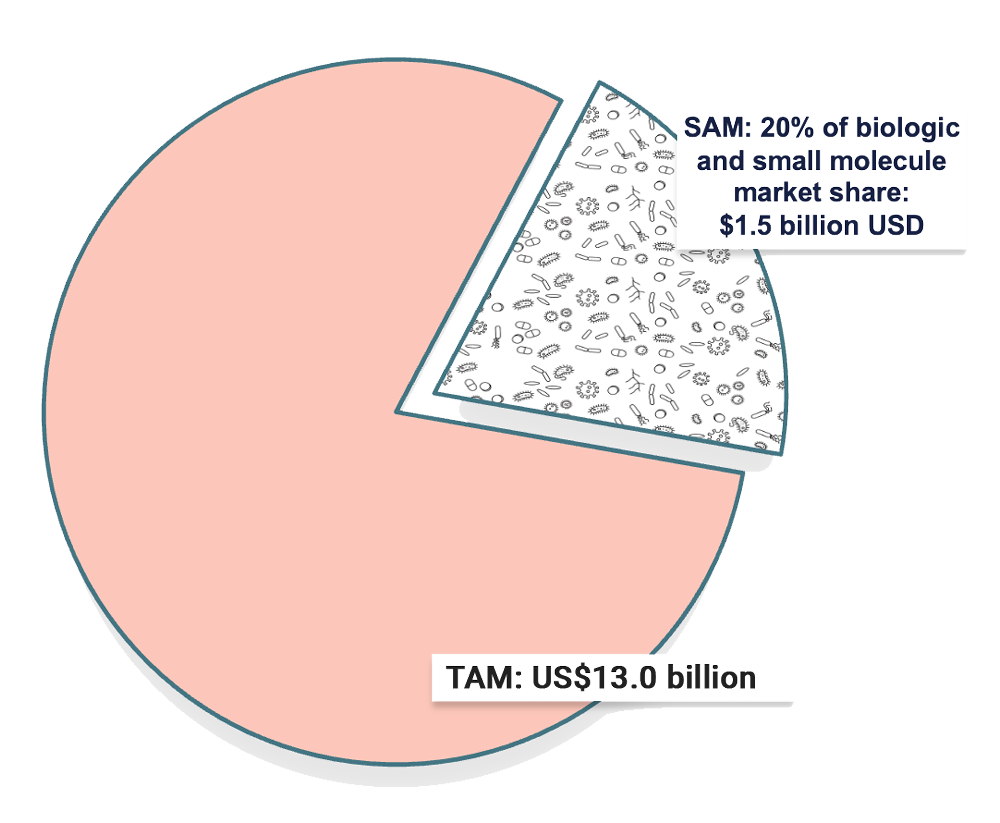
10% market penetration = $1.3 billion per year
Current therapies are inadequate with 30-35% remission rates and a 10-40% loss of response in patients. Also, many existing therapies for UC have risks of adverse events related to immune suppression, resulting in a large unmet medical need. 2
BiomeBank’s BB265 is formulated to disrupt the UC market and to provide a better solution patients with ulcerative colitis.
1. Global data UC report 2023;
2 Savelkoul et al Inflamm bowel dis 2022
Optimising
the Drug Discovery Funnel
1.
Leveraging real-world, human data from our existing therapies to establish the causation of disease, our ‘human-first’ model of discovery allows us to more rapidly and precisely identify the ‘mechanism of action’ when compared to animal models.
2.
Harnessing the latest technology, we screen the real-world data and select candidate strains from our leading culture collection. Employing our cutting-edge Consortiome platform we engineer an ecologically diverse consortia of microbes carrying that specific mechanism of action, as well as emergent therapeutic properties manifested within the whole community collectively.
3.
Delivering BiomeBank the highest potential drug candidates ready for clinical success.
The Development Pipeline
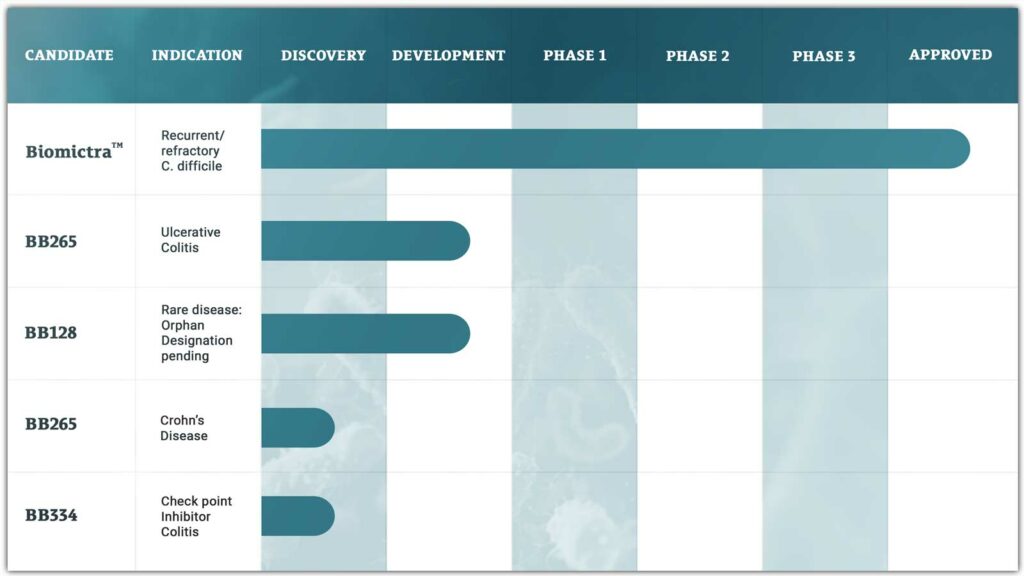
BB265
BB265 is a rationally designed cultured microbiome therapy engineered for the treatment of ulcerative colitis. BB265 was developed using BiomeBank’s Consortiome platform and data from a discovery trial using BiomictraTM (donor derived) therapy in patients with active ulcerative colitis. BB265 is scheduled to enter a phase 1b/2a study in 2025.
BB128
BB128 is a rationally designed cultured microbiome therapy engineered for the treatment of an orphan disease indication. A discovery trial in this orphan disease indication is underway using BiomictraTM.
Latest News
Get the latest information on BiomeBank, our drug development, and other important topics in microbiome science.