ConsortiomeTM:
A Breakthrough Technology
An Overview
Our team of translational microbiome experts have developed a breakthrough co-culturing platform that has enabled us to generate an artificial human gut microbial community in a bioreactor – the Consortiome™.
Consortiome™ is a rationally designed microbial consortia containing more than 90% of the known gene families that are found in a healthy person’s microbiome
Overlap of gene families between
healthy donors & BiomeBank’s Consortiome™
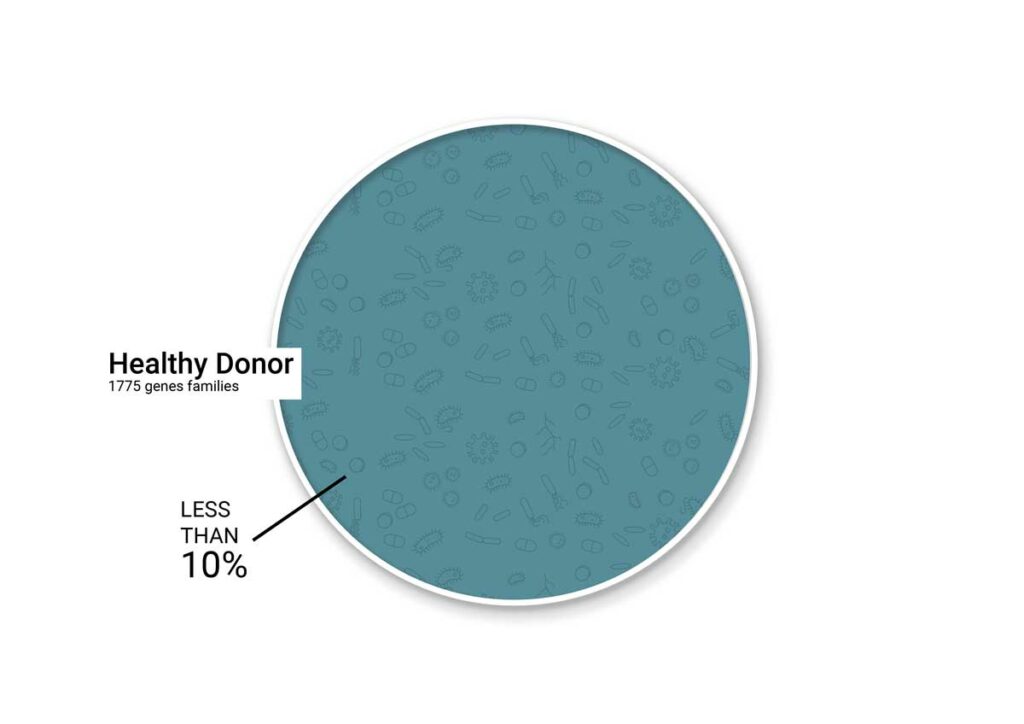
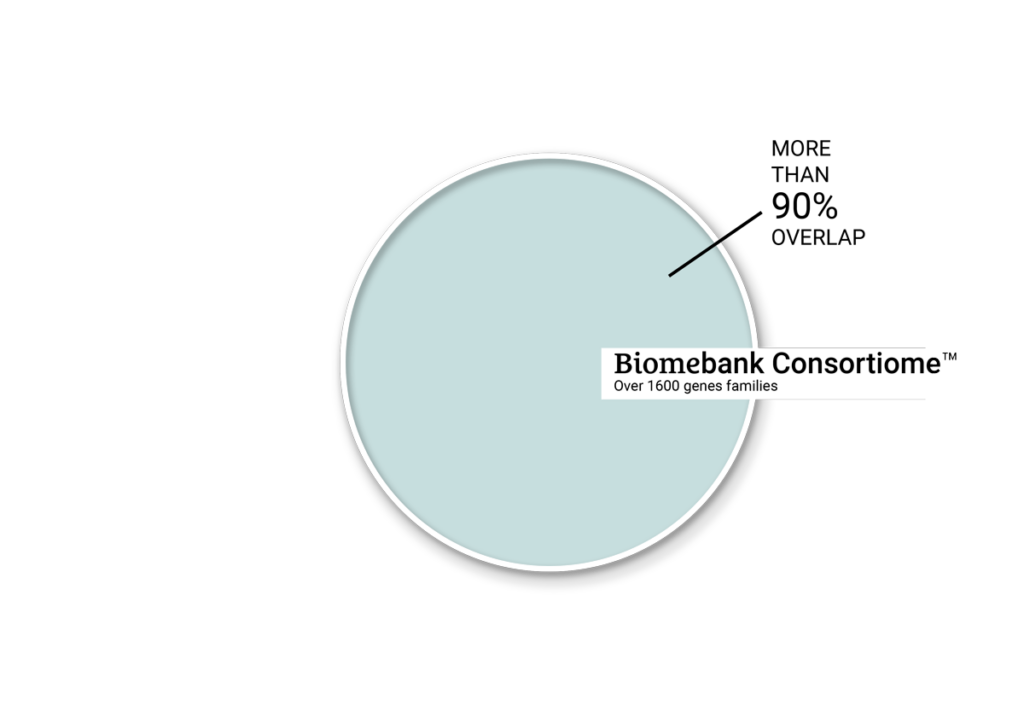
Annotation using the NCBI COG database (”Clusters of Orthologous Genes”) identified members of 1775 gene families from a healthy donor metagenome, of which over 90% of gene families were represented in the consortium metagenome.
Our first such co-cultured therapy, BB265, targeting Ulcerative Colitis and inflammatory bowel disease, has been designed using data from use of Biomictra ™ in discovery trials and will enter human trials in 2025.
Harnessing ecology and technology
to optimise the
Drug Discovery Funnel
1.
Leveraging real-world, human data from our existing therapies to establish the causation of disease, our ‘human-first’ model of discovery allows us to more rapidly and precisely identify the ‘mechanism of action’ when compared to animal models.
2.
Harnessing the latest technology, we screen the real-world data and select candidate strains from our leading culture collection. Employing our cutting-edge ConsortiomeTM platform we engineer an ecologically diverse consortia of microbes carrying that specific mechanism of action, as well as emergent therapeutic properties manifested within the whole community collectively.
3.
Delivering BiomeBank the highest potential drug candidates ready for clinical success.
The Development Pipeline
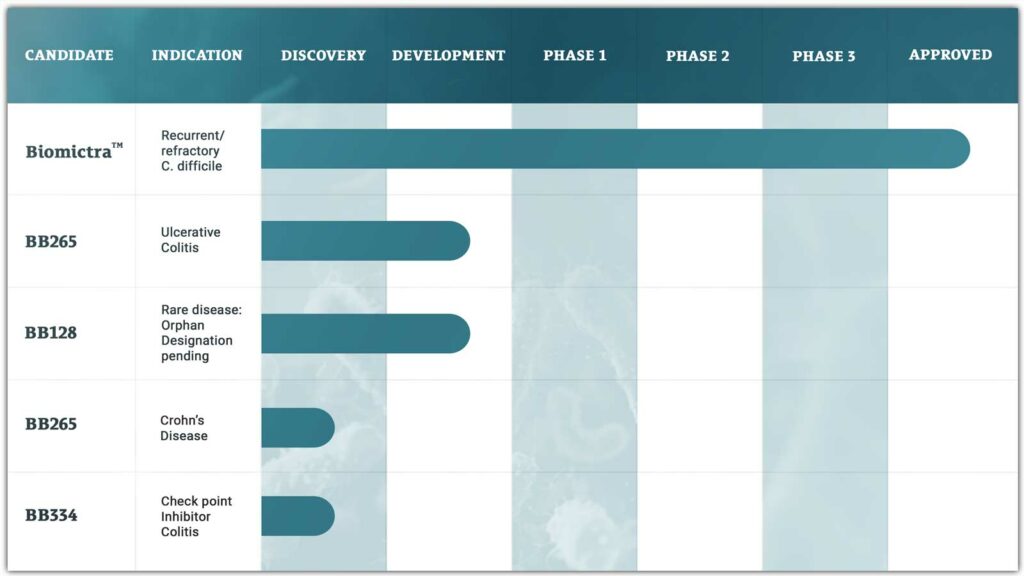
• BB265
BB265 is a rationally designed cultured microbiome therapy engineered for the treatment of ulcerative colitis. BB265 was developed using BiomeBank’s Consortiome platform and data from a discovery trial using BiomictraTM (donor derived) therapy in patients with active ulcerative colitis. BB265 is scheduled to enter a phase 1b/2a study in 2025.
• BB128
BB128 is a rationally designed cultured microbiome therapy engineered for the treatment of an orphan disease indication. A discovery trial in this orphan disease indication is underway using BiomictraTM.
